Reference no: EM131026840
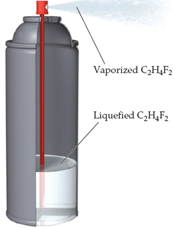
As shown here, one type of computer keyboard cleaner contains liquefied 1,1-difluoroethane (C2H4F2), which is a gas at atmospheric pressure. When the nozzle is squeezed, the 1,1-difluoroethane vaporizes out of the nozzle at high pressure, blowing dust out of objects.
(a) Based on your experience, is the vaporization a spontaneous process at room temperature?
(b) Defining the 1,1-difluoroethane as the system, do you expect qsys for the process to be positive or negative? Explain.
(c) Predict whether is ?S positive or negative for this process.
(d) Given your answers to (a), (b), and (c), do you think the operation of this product depends more on heat flow or more on entropy change?
|
When conducting a significance test to determin
: When conducting a significance test to determine if there is a difference between two treatments, with a quantitative response variable, treatments are given to different experimental units, we summarize the data
|
|
Current performance appraisal process
: 1) As the HR manager, critically evaluate the current performance appraisal process at the Financial Security Investment including Brook's manager's behavior and decision making as well as the organization's role in this process.
|
|
What is the cost for 135 minutes of calls
: Eileen is 21 years old, which is 6 less than 3 times Minnie's age in years. Write an equation to find Minnie's age. How old is Minnie?
|
|
Which type of workplace discrimination has occurred
: If a firm gives all international assignments to people without disabilities, assuming they will therefore not require special accommodations, which type of workplace discrimination has occurred?
|
|
Is vaporization a spontaneous process at room temperature
: As shown here, one type of computer keyboard cleaner contains liquefied 1,1-difluoroethane (C2H4F2), which is a gas at atmospheric pressure. When the nozzle is squeezed, the 1,1-difluoroethane vaporizes out of the nozzle at high pressure, blowing ..
|
|
How math and science has made reporting earthquakes
: Compare the Richter scale and Mercalli scale. In your opinion, explain which is more meaningful in the use of reporting earthquake destruction.
|
|
Which isomer do expect to have higher standard molar entropy
: Isomers are molecules that have the same chemical formula but different arrangements of atoms, as shown here for two isomers of pentane, C5H12. Do you expect a significant difference in the enthalpy of combustion of the two isomers? Explain.
|
|
Calculate the shortest amount of cable you need to connect
: Show manual work (by hand) step by step to reach the optimal answer (i.e., don't just provide a final answer and don't use a computer program to find a solution). Provide also a summary of your solution.
|
|
How strategic planning process can assist urgent care clinic
: Discuss how the strategic planning process can assist a primary or urgent care clinic to identify opportunities to increase the number of patients it treats.
|