Heat death of the universe - Paper
The heat death of universe is a state when the Entropy of a system goes maximum. Entropy is nothing but degree of disorder in a system, i.e., higher the entropy more the disorder in a system. From the Second law of Thermodynamics we can interpret that total entropy of any isolated system won't decrease whatever the change is. The entropy will only increase. Since the earth is considered as a closed system in thermodynamics, the entropy of the earth is keep on increasing according to the second law. At one point of time the universe will reach a state of maximum entropy, i.e., all the higher energy moves towards lower energy. When this happens work is impossible to be extracted from any source of heat because the heat stops to flow. Thus the universe will get into a state of complete equilibrium and no work can be done. So the universe will be dead and impossible for humankind to continue their survival
1) INTRODUCTION :
We don't have a clear knowledge about the universe i.e., we cannot confidently assert that none of the external forces influencing the universe or may be considered as a system in thermodynamics. By second law of thermodynamics we know that the ENTROPY of closed system always tends to maximum, since universe comes under the category of closed (isolated) system and if this was true ,then the universe entropy will reach maximum and it will attain a state of equilibrium. Then no energy like heat, electrical, mechanical would be available for purpose. This critical state is known as HEAT DEATH OF UNIVERSE. Although we don't have any proof ,this was the first concept to know the finiteness of the universe
2) LITERATURE REVIEW :
The False believe that every types of energy in the universe should finally become energy of thermal motion. Then all the energy would be distributed uniformly throughout
The universe and all big processes would come to halt.
This belief was hunched by R. Clausius by second law of thermodynamics. We know according to second law of thermodynamics ,the system existing in physical state won't exchange their energy with other systems tending towards an stable equilibrium state that is a state of maximum entropy , this state is known as the heat death of universe.
Before the development of cosmology, many attempts were done in order to disapprove the idea in the heat death of the universe. The most important among that was the fluctuation hypothesis by L. Boltzmann. By considering his hypothesis he said that the universe is already in an isothermal state but by law of chance occurrences deviations occur from that equilibrium state at times in one place or another. If a region is more encompassed by the deviations then since it has higher degree of deviation, the frequency of deviation is less
After the cosmology developed it revealed that not only the idea of the heat death of the universe but also the previous attempts to refute the idea are false. Because most important physical factors, gravitation is not considered. While we consider the effect of gravitation, the uniform isothermal distribution of matter is not possible and it won't correspond to maximum entropy. Results show that the universe is in a non-steady-state that is it's expanding stated by the big bang theory. The core which was homogeneous before expanding , broken up into individual objects as time passes, due to the action of gravitational forces, making galactic clusters, galaxies, stars, and planets. These things have happened naturally and it is not violating the laws of thermodynamics. Thus in the future too if the gravitation is taken into account it will not take universe into heat death but the universe will always expand and it is non-static.
Now we will consider the article by MattProle , he says that this heat death depends on the idea that Entropy must always increase but if we note any decrease in the entropy it is only because of increase of it at some other place in the universe. According to him if the entropy increases there will be also the decrease of free energy which is available in the universe. This is because the states of higher order possessed by the isolated system tends to move towards the lesser order and greater entropy mostly by form of heat, which cannot be easily converted into useful potential energy. He states that he has no interest in the statement of second law of thermodynamics because he was satisfied by the statistical approach , that states merely the entropy increase or making the available states more heterogeneous and less homogeneous is not an likely process in case of larger number of molecular aggregates. It is just a prediction or probability. A state when we first trap a large number of gas molecules in a room with no restriction to it, it will be gradually developing towards a homogeneous state. The reason for it is the molecules don't form a trajectory path , this can be easily understood by us , this is a description of what is going to take place instead of saying it will take place.
He also adds the discussion taking into universe describing that universe supposed to have 10^80 atoms. This atom has expanded from a state of very high density during of which the universe has been made with a solar system, and life. But if the universe consistently expands then it will increase its entropy along with its potential energy which is sustaining the human life on it. From the second law of thermodynamics we can also know it is impossible for our universe to again aggregate into higher lower state which means universe will die.
But most important think to be noted from his article is he states that saying impossible to decrease the entropy is an erroneous assumption. If we look at it from statistical point of view we would come to that we could potentially get back to the lower entropy state if very large enough time is given for the change to take place. At last he concludes that if we give enough time the entropy will decrease and also substantially it will make universe a place of life.
3) BIG BANG THEORY :
The origin of universe is explained by the big bang theory. This theory states that the universe is formed by expansion of hot dense state and it also expands today too. This theory explains the origin of universe from its beginning. The big bang of universe according to this theory is happened 13.77 billion years ago. Hence after that the universe started to expand and release the subatomic particles like protons neutrons and electrons.
We don't know from where and how did our universe formed. But they are thought to be in a form of core of black holes commencing into existence 13.7 billion years ago. Those black holes are intense gravitational pressure areas. That pressure is so intense to make that finite matter into infinite density. This infinite zone is known as singularities. This after its appearance began to expand and then cooled and again expanded from very tiny size to size of current universe. It haven't stopped and it continues to expand and cool consistently having all the human beings inside it, also having huge amount of magnificent stars which all came from a unknown small infinite dense core.
COMMON MISCONCEPTIONS IN BIG BANG THEORY :
? First one is everyone does think that there was an explosion but actually the scientist say that there wasn't any explosion but only expansion from a small ball sized shape to the shape it is now
? One more is the singularity mentioned above is thought as the already born in space but it is wrong. Many experts say that the space hasn't formed before the big bang. The three British scientists, Steven Hawking, Roger Penrose and George Ellis, published paper related to notions of time by which they extended the concept of the Einstein's Theory of relativity . By their calculations they came to a conclusion that the time and space appeared only after the singularity i.e., from the singularity. From which came the time energy space etc., before that nothing existed. But yet the mystery about how the singularity has been formed is unsolved
4) BIG BANG TO HEAT DEATH:
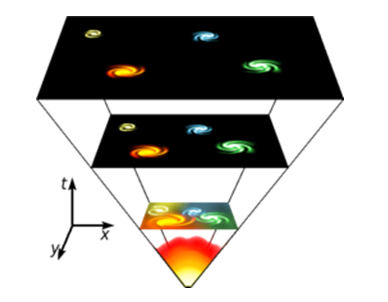
Graphically depicts the timeline of universe from bigbang to Heat death.
5) SECOND LAW OF THERMODYNAMICS :
We know that the first law of thermodynamics is the energy conservation principle. For a process to occur it must satisfy the first law of thermodynamics. But that alone is not enough to say that a process is feasible or not.
Now this can be understood by taking the following example, consider a cup of hot tea placed on the table, the tea will get cooled by losing heat to the surrounding air, by first law the energy lost by the tea is gained by air and hence the energy is conserved. But the same can't be reversed that is the tea won't or can't get heat from the surrounding air even though the first law gets satisfied. Thus the process is not reversible. Also we can come to a conclusion that first law alone is not enough to predict the process occurrence we also need another law to predict it. So there comes the second law which gives the possible direction of the process. So a process can occur only if it satisfies both the first and the second law of thermodynamics. The violation of the law can be easily identified by the property called entropy. The second law of thermodynamics is also used to know the efficiency of the systems like heat engines, refrigerators.
In the second law a most important thing is a thermal energy reservoir, that is an imaginary body with high thermal capacity and it can supply heat without reduction in its temperature. For example our atmosphere is a thermal reservoir which doesn't get warm up because of heat losses from the surrounding during winter. Like the same oceans, rivers are all bodies with large theral capacity.
A reservoir that supplies heat is known as source and the one that absorbs energy in the form of heat is known as sink. In order to transfer heat into work the device known as heat engines are found.
There are two main statements related to the second law of thermodynamics, they are
? Kelvin-Planck Statement
? Clausius Statement
KELVIN-PLANCK STATEMENT:
It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work.
It states that a single reservoir heat source can't do the complete cycle producing work , a lower temperature sink is required to make the flow of heat. From this we can also know that no heat engine produces 100%efficient energy some energy is rejected out always. This statement is for heat engines
CLAUSIUS STATEMENT:
It is impossible to construct a device that operates in a cycle and produces
no effect other than the transfer of heat from a lower-temperature body to a
higher-temperature body.
This statement is related to the heat pumps and refrigerators. It states that heat can be transferred from the low temperature sink to high temperature reservoir only if an external work is given to it unless the heat transfer is impossible.
6) ENTROPY :
What is entropy? The mostly heard answer for this question is that it is a kind of measure of order. But this is wrong. When we equate entropy with disorder it will create chaos in entropy of different systems. Let's take example of following cases
? A bunch of play cards in exact order or the same in random order
? 10 ice blocks kept on a table or the same equivalent mass of water in a bottle kept at some place in space
? Our universe at the beginning of the Big Bang or the universe at the present now
If we consider entropy as a disorder then the answer to the following questions will be a trouble
ENTROPY ACCORDING TO THE CLASSICAL THERMODYNAMICS:
If we look for the origin of the entropy, its concept is actually originated from mid-century by the study of heat, work, energy and temperature, which is known as thermodynamics . That period is steam engine Era and interest of people is around converting heat into mechanical work. They found that there is a relation between the heat and temperature, the more an object gets heated higher the temperature. They found that heat always flow from hot body to cold body and their objective is to find a way to extract work from the flow of heat
One of trouble during their analysis is they couldn't find the amount of heat content in the reservoir or to know how much heat could be extracted from the reservoir. Thus the heat content couldn't be measured directly they were able to measure only the temperature of the reservoir. So if we know a relation between the temperature and the heat content we can easily know the heat content in reservoir. If the temperature is reduced to zero then the heat content is also reduced to zero so there is a ratio relationship between them which will be ratio that is defined as ENTROPY. Entropy is simply relation between the heat content and the temperature
S=Q/T
S=Entropy
Q=Heat stored
T=temperature of object
SIGNIFICANCE OF ENTROPY IN CLASSICAL THERMODYNAMICS:
Thus as we already discussed above the significance of the entropy in these heat engines is to find the amount of heat content in the system depending on the temperature. If there is a change in entropy of a system somewhere a similar energy would have released or absorbed. That is when converting heat into work by passing it into a cold reservoir, we have to look for the cold reservoir capacity such that it doesn't get heated up nor the hot reservoir should lose its heat energy. So if we maintain this temperature difference than heat will flow continuously producing mechanical work. So we need entropy in order to maintain this
7) NATURE OF ENTROPY:
From the second law of thermodynamics we always get lot of inequalities. A process which is irreversible is always less efficient than a reversible one. Another important inequality that has major consequences in thermodynamics is the Clausius inequality, stated by the German physicist R. J. E. Clausius (1822-1888),one of the founders of thermodynamics, and is expressed that the cyclic integral of dQ/T is always less than or equal to zero. This is valid for the reversible and also irreversible process
Now let's look on the relation of entropy, we know that the cyclic integral of work is not zero, if it is we can't get any work out of heat engines working in cycle. For heat also the cyclic integral is not zero . But now let's take example of a cylinder with volume of gas in it. After the expansion and returning back to original position, there is no change in the volume and hence its cyclic integral is zero. A quantity which has cyclic integral zero depends upon state only and it is a property since it doesn't follow property path. Thus the ENTROPY is a property and can be expressed as
dS = (δQ/T)int rev (KJ/K)
The entropy change for a process or a cycle can be determined between the two states S1 and S2 by the following expression
ΔS = S2 - S1 = 1∫2 (δQ/T)int rev
THE INCREASE OF ENTROPY PRINCIPLE :
In order to understand this lets take an example of two process 1-2 and process 2-1 ,
Which are internally reversible.
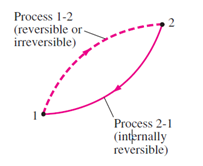
From the Clausius inequality,
∫ (δQ/T) <= 0
Or
1∫2 (δQ/T) + 1∫2 (δQ/T)int rev
The second integral in the previous relation is recognized as the entropy
change S1 _ S2. Therefore,
1∫2 (δQ/T) + S1- S2 <= 0
which can be rearranged as
S2 - S1 = 1∫2 (δQ/T)
It can also be expressed in differential form as
dS >= δQ/T
This above equality is valid only for internally reversible process also for an irreversible process of inequality. From this we can conclude that entropy change of a closed system during an irreversible process is greater than the integral of dQ/T evaluated for that process. Also the T in the above relation indicates thermodynamic temperature at the boundary and the dQ is the differential heat that is transferred between the system and surroundings
The quantity S2-S1 represents the entropy change of the system . This change becomes equal to for an reversible process and this represents entropy transfer with heat . In the preceding relations the inequality sign is a constant indicating that the entropy change of a closed system Is always greater than the entropy transfer during an irreversible process. Because of the presence of irreversibility's entropy is generated in an irreversible process. This entropy generated is known as ENTROPY GENERATION and it is denoted by Sgen . By considering the variation between the entropy change of a closed system and also the entropy transfer is equal to entropy generation , so the equation
S2 - S1 >= 1∫2 (δQ/T)
can be written as
ΔSsys=S2 - S1 = 1∫2 (δQ/T) + Sgen
The entropy generation Sgen is always a positive value i.e., greater than or equal to zero. It is not a property of the system as its value depends on the process. Also if there is no entropy transfer the entropy change of system is equals to entropy generation
So for an isolated system the equation can be written as
S2 - S1 >= 1∫2 (δQ/T)
ΔSisolated >= 0
The above equation gives the entropy of an ISOLATED SYSTEM during a process it always increases. And main thing to note it never decreases, instead it will be constant during a reversible process. This is known as increase of entropy principle
Sgen > 0 IRReversible process
Sgen = 0 Reversible process
Sgen > 0 Impossible process
Thus the entropy of a process always increases until reaching a maximum point which is known as state of equilibrium. This is the state of HEAT DEATH OF UNIVERSE
8) ORDER:
The term order means that having everything properly also behaving in proper manner. Disorder is opposite of that, nothing will go in right manner. Order is flight arriving in time , reaching correct place on schedule . Order is employee's performing the job correctly according to the instructions if their managers. A machine which doing its intended job correctly is order and if doesn't do so then the machine is out of order.
Also if we state order as seen above it doesn't only mention or refer moving things , it even refers to doing right things sitting in a place, or even placing things in a place in a manner like storing the books in a shelf. Thus an order can be static or dynamic. This order was only considered in entropy as it is in a state of order or disorder.
9) ORIGIN OF ECOLOGICAL ECONOMICS:
Ecological economics came to existence in 20th century in order to protect environment and sustainability of the economic. It is a disciplinary which incorporates the concepts and innovations from natural and social sciences. In this important ones are first law of thermodynamics and ecology principles. The extent which the economy grow can be known from the first two laws of thermodynamics. By the first law we can know that there is limits in giving input for economic growth and from the second law we can know the efficiencyof the process , that is how much output we can get from the given input . Since it comes from the natural science its main aim is for allocation and distribution of the resources. In the latest economics the main concern is for to allocate the resources efficiently. For the allocation of the labor, capital in order to maximize production also to increase the growth of economics. For efficient allocation of resources certain ideas are all have been used. If the universe has been lead to the heat death then entire economics will be unpredictable
10) CONCLUSION:
In the universe we aware of the Black holes. This is major threat for its destruction. Still
The scientist is not clear of what had happened before the BIG BANG and how everything came from nothing. Think of if our universe is from a black hole it will be odd to hear but it will be a good explanation for how the universe formed. It is been said that the universe had come from a very dense infinite particle and expanded but many important questions are still unanswered like,
? Because of what Big bang started?
? What had caused the inflation i.e., the expanding?
? What is the mysterious energy that is still making the universe to expand?
With these unanswered questions we can't be sure about heat death, but one thing if the entropy is increasing; it is also to be noted that the universe also equally expanding.