Capturing and use of carbon dioxide?
Introduction
With the increase in the world population and increase in the industrialization the demand for energy is increasing rapidly. Presently over 85% of the world energy needs are coped-up by using fossil fuels. By using these fossil fuels there is emission of carbon dioxide into the atmosphere due to which there is increase in the CO2 concentration in the atmosphere which create an imbalance in the carbon concentration in the atmosphere. Since the beginning of industrial revolution the amount of CO2 in earth's atmosphere increases from 280 to 390 ppm [1]. This increase in the CO2 concentration leads to disturb the incoming and outgoing energy balance which finally leads to the rise in average temperature of the earth's surface. Thus, CO2 is considered as the leading anthropogenic green house gas. However, in order to reduce the emission of carbon dioxide in the environment there is need of hour to find the alternative clean fuels to replace fossil fuels. In addition to the replacement of these fossil fuels efforts are being made across the world to reduce the carbon dioxide concentration from the atmosphere. Different methods have been employed by human beings to capture carbon dioxide and then utilize it in different ways.
Capturing of Carbon dioxide
Figure 1 is the pictorial representation of different options for carbon dioxide capture produced from power generating plants
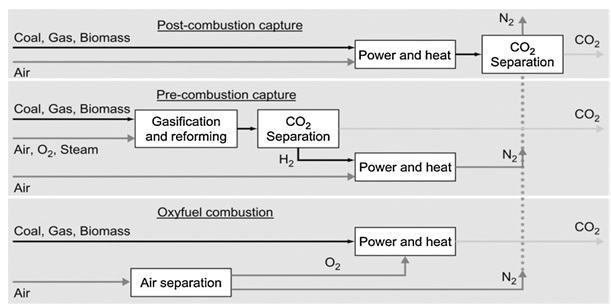
Humans have developed many technologies for carbon dioxide capture. But, naturaly carbon dioxide is captured by green plants and uses it for photosynthesis in presence of sunlight and water. During the photosynithesis carbon dioxide is taken from the atmosphere from this process oxygen is replinsed into the air which is being used by almost every living being. Based on the carbon dioxide generation, several capture process and technologies have been developed and implemented. Depending on the chemical process involved in the carbon dioxide production during fossil fuel burning, three methods have been evolved to capture the carbon dioxide (1) pre-combustion capture; (2) oxy-fuel combustion; and (3) post-combustion capture [1]. The three options are schematically represented in figure 1.
1. pre-combustion capture:- in this capture there occurs the reaction between primary fuel and oxygen or air to produce H2. In certain reactions the gas which is produced is mainly composed of CO and H2, which is also known as syngas or synthetic gas. The CO(carbon monoxide) which is produced here is further reacted with steam in a catalytic reactor to produce more H2 along with carbon dioxide. The carbon dioxide and H2 gas produced during the reaction are separated by using different technologies. There are various advantages as well disadvantages of this pre-combustion capture like main advantage involves lower energy requirements but the temperature and efficiency associated with this method is a big disadvantage. An alternative to produce the syngas involves chemical looping which involves less use of capital.[7]
2. oxy-fuel combustion:- this process of carbon dioxide capture requires pure oxygen instead of air and the carbon dioxide produced is of high purity that can be stored directly. The main drawback of this technique is the requirement of pure oxygen which inturn is produced by using highly sophisticated techniques and the use of these techniques to produce pure oxygen requires high capital cost.
3 post-combustion capture:- in this process of CO2 capture there requires removal of carbon dioxide before emission into atmosphere from flue gases which usually comprises of CO2 and Nitrogen. On short time scale post combustion treatment is most feasible process for carbon dioxide capture, because many of the substances can be added to the fossil fuel used by power plants and these substances can reduce the emission of carbon dioxide into the atmosphere. One of such methods involves the feeding of cooled CO2 rich flue gases into the bioreactor to produce microalgal biomass which can be latter used as biofuels. One main advantage of this post combustion capture is if the carbon dioxide unit is shut down due some reason we can still generate electricity from this process.
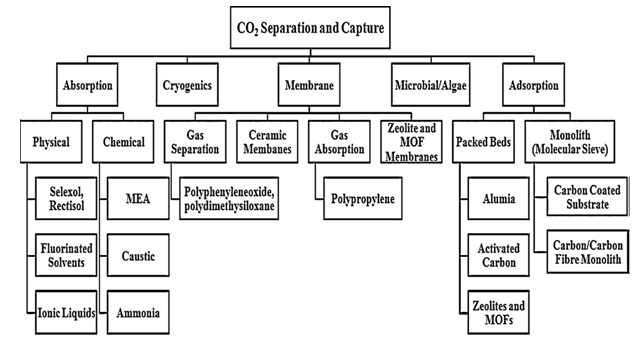
In addition to above three processes another important process of carbon dioxide capture is adsorption. Adsorption is well established process for carbon dioxide capture used in chemical and petroleum industries. Adsorption is further divided into two categories 1. Physical adsorption. 2. Chemical adsorption. In physical adsortption there is no formation of chemical bonds and is temperature and pressure dependent. This type of absorption occurs at high pressure and low temperature. In chemical adsorption the adsorption of carbon dioxide depends upon the chemical reaction taking place between the adsorbate (adsorbing substance) and the adsorbant /carbon dioxide and also depends on acid base neutralization reaction. In addition to all these techniques there are vast number of technologies adopted for carbon dioxide separation and capture and figure 2 is the pictorial representation of these different technologies used for carbon dioxide separation and capture
Fig. 3. Different technologies and associated materials for CO2 separation and capture
Uses of carbon dioxide capture:-
Prime and important use of carbon dioxide capture is the reduction of its concentration into the atmosphere because increased concentration of carbon dioxide has resulted in grave environmental pollution. In addition to control the environment pollution its capture by plants during photosynthesis is an important source of food and energy to the human beings and many other living beings. As discussed above the capture of carbon dioxide leads to the production of H2 gas and this H2 gas is being considered as a valuable alternative source of energy for fossil fuels. The stored carbon dioxide is also used in oil industries for the enhancement of oil production from the mature oil fields. This enhancement of oil recovery involves mixing of carbon dioxide with the crude oil which results in the swelling of crude oil and reduces the viscosity which helps in maintaining or increasing the pressure in the reservoir. Due this combination process there occurs more flow of crude oil to the production wells. As discussed above captured carbon dioxide is also used in formation of biomass which later can be used as biofuels.