Silicon Crystal with a Si Atom:
In pure (intrinsic) semiconductor, the electron concentration in the CB and hole concentration in the VB are few compared to the number of available energy states. Adding impurity atoms called dopants may increase this concentration and the procedure is known as doping. There are two types of dopants : donors and acceptors. A donor has more electrons for bonding with the neighbouring atoms. For example, Phosphorous (P) has five electrons, which may make boding while Silicon has only four electrons for bonding. If we replace a Si atom by a P atom in the Si lattice then an electron shall be free for the P atom and it will donate it to the CB as shown in Figure (a). An acceptor has fewer electrons for bonding than the neighbouring atoms. For instance, Boron (B) has 3 electrons for bonding and if we replace a Si atom in the Si crystal with a B, then a hole shall be created in the VB as illustrated in Figure (b). A semiconductor doped with donor is called a n-type semiconductor and a semiconductor doped with acceptor is called as p-type semiconductor. The majority carriers in n-type semiconductors are electrons and in p-type semiconductors are holes.
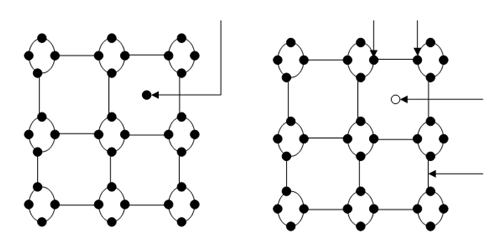
(a) (b)
Figure: Silicon Crystal with a Si Atom Replaced by (a) Phosphorous Atom; and (b) Boron Atom