The design of a monolith reactor for the VOCs removal.(Benzene)
Therefore, you are asked to do the following:
1. Choose a specific VOC;
2. Search for the reaction rate for the catalytic combustion of that VOC;
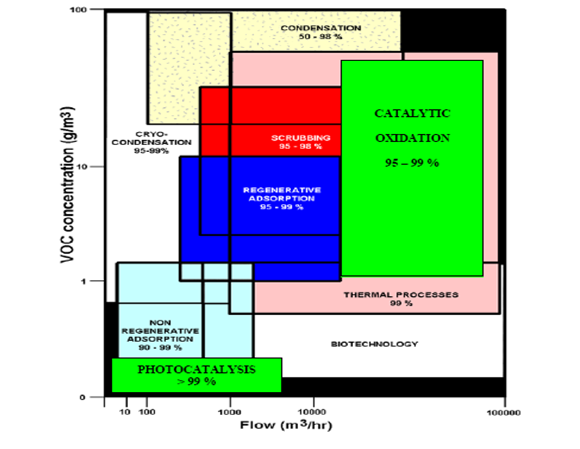
3. Pick the concentration and flow rate from the graph provided within your lecture;
4. Design a monolith reactor to address the VOC removal, assuming a 99% efficiency.
Your report will contain:
1. Introduction: identify the industrial flue gas which would release the chosen VOC, short description of the process and reasons for the use of catalytic incineration for air purification;
2, Monolith reactor model and design approach;
3, Results: reactor volume and optimal configuration, graphs showing the conversion and temperature variations along the monolith wall;
4. Conclusions.