Age-hardening of Aluminium Alloys
In specific alloys precipitation from a single phase may arise. The precipitate phase might be in form of determine sub-microscopic particles distributed both around the grain boundaries and via the grains. In specific alloys of Be-Cu, Al-Cu and Mg-Si that type of phases precipitate after suitable heat treatment. Such precipitated phases comprise strengthening the alloys effects. That hardening of alloys is known as precipitation hardening or age hardening.
Now the process of age-hardening will be illustrated with particular reference to aluminium alloys containing 4% copper. Following figure depicts the equilibrium demonstration of Al-Cu system. This is seen that the solubility of Cu in a-phase solid solution reduces steadily and slightly considerably along with decrease in temperature. On temperature consequent to point 3, Cu forms copper aluminide or CuAl2 such is deposited like coarse particles in and around the grains of a-solid solution. CuAl2 is extremely hard and brittle. Whether the alloy is now reheated to about 550oC, in between the points 2 and 3, CuAl2 is reabsorbed in a-solid solution resulting in single-phase alloy. Whether alloy from such state is quenched to room temperature, there is inadequate time for CuAl2 to form and copper atoms are here held in a super-saturated solid solution inside the aluminium.
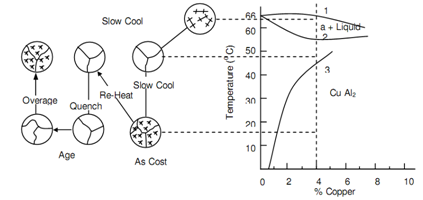
Figure: The Aluminium-rich Portion of the Copper Aluminium Equilibrium Diagram demonstrating the Mechanism of Precipitation hardening for a 4 percent Copper Alloy, Over Aging origins a Coalescence of the CuAl2 Particles and consequential Loss of Strength in the Alloy
While this alloy is allowed to stay at room temperature for five to seven days, the strength enhances significantly due to slow precipitation of get submicroscopic particles. These particles are around the grains, mostly uniformly distributed. The time of this precipitation may be decreases to a few hours with heating the quenched alloy to 120oC. This is called as artificial age-hardening. Both time, closed control and temperature is important in precipitation hardening for this reasons. Salt baths at constant temperatures are utilize 4% Cu aluminium alloy is most suitable for such type of treatment. Though, this alloy loses its corrosion resistance in hardened state and should be protected by cladding.
Age-hardening alloys comprising Si and Mg behave in a same manner. The submicroscopic particles however, provide strengthening that are made of magnesium silicide or Mg2Si. Hence the age-hardening effect of CuAl2 is reinforced via Mg2Si.