Reference no: EM131347885
Another diatomic ideal gas-entirely classical this time, but in two dimensions
Consider a classical, dilute, diatomic gas in two dimensions. The gas is again ideal in the sense of neglecting interactions between the molecules. Each molecule consist of two point masses. Although there are no interactions between different molecules, there is an interaction between the atoms in the same molecule of the form
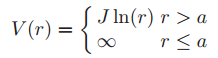
The gas is contained in a two-dimensional ‘box' of area A = L2. The whole system is in equilibrium with a thermal reservoir at temperature T.
1. What is the classical Hamiltonian for this system?
2. Write the canonical partition function as an integral over phase space.
3. Calculate the partition function in closed form, under the assumption that the molecule is much smaller than the box it is in. That is, let the limits on the integral over the separation between the atoms in a molecule extend to ∞.
4. This model is only valid for low temperatures. At what temperature do you expect it to break down?
5. Now calculate the average square separation r2 between the atoms in a molecule.