Reference no: EM131041167
1. a. How many radial nodes arc present in the radial eigenfunction associated with the 2p atomic orbital for hydrogen-like systems? What about the number of nodes in the corresponding spherical harmonics?
b. For the hydrogen-like Mercury ion (Min, at what values of r will the radial nodes appear?
Note: R21(r) = {1 / 4(6)1/2}{Z/aa}3/2 ρe-ρ/4
Where ρ = 2Zr / aa
2. Using the atomic wavefunctions given in class and in your textbook, plot BY HAND the probable location of the electron contained in the 2p] orbital of a hydrogen atom on the graph below. Explain with the help of equations, how you achieved this plot?
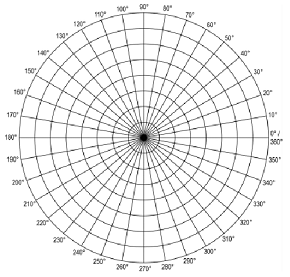
3. The normalized eigenfunctions for a particular hydrogen-like atom whose radius is Ro are
Ψ(r, θ, Φ) = Rn1(r) Y11(θ, Φ) for r ≤R0
and Ψ(r, θ, Φ) = 0 for r>R0
Y11(θ, Φ) is a spherical harmonic equal to (3/8 π)1/2 sin θei θ, and Rn0(r) = (2/R0)1/2[(sin [nπr/R0])/R0]
What is the average radial position of the particle as a function of the quantum number n.
|
How does the process of factoring work
: 1. How does the process of factoring work? 2. What would be the impact on the company you wrote your research paper on if they began to use some form of factoring for all or some receivables? or how does factoring they already use help them?
|
|
Discuss what growth strategies you would focus on
: Discuss the resources you would need to implement the marketing plan as it relates to the following: services you would include for your target market, how you would achieve seamlessness, and how you would promote organizing access.
|
|
Cumulative voting procedures
: If the company uses cumulative voting procedures, how much will it cost to guarantee yourself one seat on the board of directors?
|
|
Discusses performance-based trends in patient safety
: You are required to use and cite a minimum of two references from the GCU Library to support your response.
|
|
What is the average radial position of the particle
: What is the average radial position of the particle as a function of the quantum number n. How many radial nodes arc present in the radial eigenfunction associated with the 2p atomic orbital for hydrogen-like systems?
|
|
Reading assignments throughout the semester
: Your In the News assignment is designed to get you thinking critically about the reading assignments throughout the semester. Select a news story that you read in a magazine (e.g., Time), newspaper (e.g., Wall Street Journal), or online news sour..
|
|
The total cost of the existing system with one service crew
: A crew of mechanics at the Highway Department garage repair vehicles that break down at an average of λ = 8 vehicles per day (approximately Poisson in nature). The mechanic crew can service an average of μ= 11 vehicles per day with a repair time dist..
|
|
What common factors lead to the overlap
: Does the structure work for the organization you selected? Why or why not?You are required to use and cite a minimum of two references from article to support your response.
|
|
Dilemma of teamwork-individual work
: Discuss how the dilemma of teamwork vs. individual work might be intensified in a virtual team. What dilemmas do you encounter when you have to do class assignments as part of a team? Discuss
|