Reference no: EM131102175
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question
1) The combustion of propane produces carbon dioxide and steam.
C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g)
All of the following statements concerning this reaction are correct EXCEPT
a. three molecule of carbon dioxide are formed per one molecule of propane consumed.
b. five molecules of oxygen are consumed per one molecule of propane consumed.
c. four moles of steam are formed per five moles of oxygen consumed.
d. the combined mass of reactants consumed equals the mass of products formed.
e. three grams of carbon dioxide are formed per five grams of oxygen consumed.
2) Atomic orbitals extending over two atoms or more provide an explanation for:
a. ionic bonding.
b. resonance.
c. covalent bonding.
d. double bond.
3) What are the bond angles in the following molecular model of BCl3?
a. less than 109.5
b. 109.5°
c. less than 120° but greater than 109.5°
d. 120°
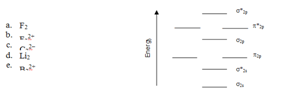
4) According to molecular orbital theory, which of the following species has the highest bond order? (you may use the diagram here as a guide)
5) If 136 J is required to change the temperature of 8.75 g of nickel by 35.0 K, what is the specific heat capacity of the metal?
a. 0.0294 J/g•K
b. 0.311 J/g•K
c. 0.417 J/g•K
d. 0.444 J/g•K
e. 2.25 J/g•K
6) The N2H2 molecule has the Lewis structure shown below. Which of these statements is correct?

a. the double bond is part of a resonance structure;
b. the bond between the two N atoms is a combination of a sigma and a pi bond;
c. the shape of the molecule is linear;
d. the angle H - N = N is 109 degrees.
7) Which of the following figures represents 3H ? Unshaded spheres represent neutrons and shaded spheres represent protons
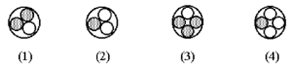
a. figure (1)
b. figure (2)
c. figure (3)
d. figure (4)
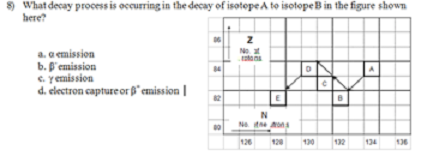
8) What decay process is occurring in the decay of isotope A to isotope B in the figure shown here?
 9) The HCN molecule has:
9) The HCN molecule has:
a. One single bond between H and C, three single bonds between C and N;
b. Two double bonds between all atoms.
c. One single bond between H and C, and one triple bond between C and N;
d. One sigma bond between H and C, and a pi bond between C and N.
10) Based on periodic trends, which of the following elements has the lowest electronegativity?
a. Cl
b. Ca
c. Mg
d. Br
11) Which one of these statements related to the nuclear fuel cycle is incorrect:
a. storage is a short-term option, disposal is permanent;
b. recycling of spent fuel is an alternative to waste disposal;
c. a volunteer community is being sought to host a disposal facility;
d. deep geological disposal was judged inadequate by the Canadian public.
12) How many structures (i.e., unique isomers) exist for dichlorobenzene?
a. 2
b. 3
c. 4
d. 5
e. 6
13) What is the oxidation number of the chromium atom in K2Cr2O7?
a. +6
b. -2
c. +7
d. +2
14) What is the shape of the following molecular model of TeF5-?
a. trigonal bipyramidal
b. octahedral
c. square pyramidal
d. square planar
15) If the age of the Earth is 4.5 billion years and the half-life of 40K is 1.26 billion years, what percent of the Earth's original amount of 40K remains today?
a. 8.42%
b. 12.1%
c. 14.0%
d. 28.0%
16) What is the Formal Charge on the carbon atom in the carbonate ion?
a. 0
b. +1
c. -2
d. -1
17) How many valence electrons are there in the arsenate ion (AsO3-)?
a. 1
b. 23
c. 24
d. 18
18) Knowing that the nuclear "magic numbers" are 2, 8, 20, 28, 50, 82 and 126, which of the following elements is expected to be particularly stable?
a. 210 Pb
b. 14 N
c. 12C
40
20
19) Which combination of atoms has the highest covalent character?
a. Al and Br
b. Na and S
c. Ca and O
d. Si and C
e. Li and F
20) In the reaction of sodium metal with chlorine gas, which of the following processes represents the electron affinity?
a. Cl2(g) → 2 Cl(g)
b. Na(g) → Na+(g) + e-
c. Cl(g) + e- → Cl-(g)
d. Na(s) → Na(g)
21) The point of maximum stability in the binding energy curve occurs in the vicinity of which one of the following isotopes?

23) Which of these molecules contain a triple bond?
a. O3
b. F2
c. CO
d. H2CO
24) Which one of the following combinations of neutrons/protons results in the highest number of nonradioactive (stable) isotopes?
a. even number protons/even number neutrons
b. odd number protons/odd number neutrons
c. odd number protons/even number neutrons
d. even number protons/odd number neutrons
25) Which molecule in the reaction below undergoes oxidation?
2 C2H2(g) + 5 O2(g) ? 4 CO2(g) + 2 H2O(g)
a. C2H2
b. O2
c. H2O
d. CO2
e. This is not a redox reaction
26) For the deep geological concept for the disposal of radioactive waste, the aim is to:
a. hide the wastes from visibility and forget about them;
b. fill the mine where the material came from originally;
c. provide a secure place where the wastes can be retrieved for future re-processing;
d. isolate radiotoxic wastes from the biosphere and provide a long travel path.
27) Which of the following ions have the same ground state electron configuration: Sn4+, Pb4+, Sr2+, and Br-?
a. Sn4+ and Pb4+
b. Pb4+ and Sr2+
c. Sr2+ and Br-
d. Pb4+ and Br-
e. Sn4+, Sr2+, and Br-
28) A silicon atom has valence electrons.
a. 0
b. 2
c. 4
d. 6
e. 10
29) How many sigma (σ) bonds and pi (π) bonds are in the following molecule?
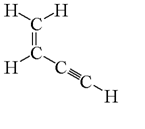
a. five σ and two π
b. five σ and three π
c. five σ and five π
d. seven σ and two π
e. seven σ and three π
30) Calculate ΔHof for sulfur dioxide, S(s) + O2(g) → SO2(g), given the thermochemical equations below.
2 S(s) + 3 O2(g) → 2 SO3(g) ΔHr = -791.5 kJ
2 SO2(g) + O2(g) → 2 SO3(g) ΔHr = -197.9 kJ
a. -296.8 kJ/mol
b. -395.7 kJ/mol
c. -494.7 kJ/mol
d. -593.6 kJ/mol
e. -989.4 kJ/mol
31) Covalent bonding is a:
a. transfer of electrons.
b. sharing of electrons.
c. gain of electrons.
d. loss of electrons.
32) The addition of water to ethylene has become a cheaper method than fermentation for making .
a. ethanol
b. antifreeze
c. diethyl ether
d. methanol
e. formic acid
33) In which of the following molecules and ions does the central carbon atom have sp
hybridization: Cl2CO, CH2Br2, CO2, and OCN-?
a. Cl2CO only
b. Cl2CO and CH2Br2
c. CH2Br2 and CO2
d. CH2Br2 and OCN-
e. CO2 and OCN-
34) If 2.891 g MgCl2 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the magnesium chloride solution?
36) Which of these examples corresponds to an octahedral arrangement (bond and/or electron cloud)?
a. IF5
b. IF4+
c. SF4
d. I3-
37) Which of the following chemical equations corresponds to the standard molar enthalpy of formation (ΔHof) of N2O?
a. NO(g) + 1/2 N2(g) → N2O(g)
b. N2(g) + 1/2 O2(g) → N2O(g)
c. 2N(g) + O(g) → N2O(g)
d. N2(g) + O(g) → N2O(g)
e. 2 N2(g) + O2(g) → 2 N2O(g)
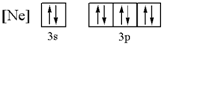
38) What 3- ion has the following ground state electron configurationa. oxide ion
b. potassium ion
c. sodium ion
d. aluminum ion
e. phosphide ion
39) Which of the following chemical equations refers to the second ionization of Mg?
a. Mg(s) + 2e- → Mg2-(s)
b. Mg(s) → Mg+(s) + e-
c. Mg(g) → Mg2+(g) + 2e-
d. Mg+(g) → Mg2+(g) + e-
e. Mg2+(g) + e- → Mg+(g)
40) Which of these ionic solids would have the highest lattice energy?
a) NaCl
b) CsF
c) MgBr2
d) CaO
e) Ba(NO3)2
41) Iron reacts with hydrochloric acid to produce iron(II) chloride and hydrogen gas.
Fe(s) + 2 HCl(aq) —> FeCl2(aq) + H2(g)
What mass of H2(g) is produced from the reaction of 5.2 g Fe(s) with excess hydrochloric acid?
a. 0.094 g
b. 0.19 g
c. 5.2 g
d. 6.8 g
e. 1.4 × 102 g
42) Sulfur hexafluoride is produced by reacting elemental sulfur with fluorine gas.
S8(s) + 24 F2(g) —> 8 SF6(g)
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
a. 40.2%
b. 45.8%
c. 47.6%
d. 54.6%
e. 61.0%
43) If 8.19 g KIO3 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the potassium iodate solution? The molar mass of KIO3 is 214 g/mol.
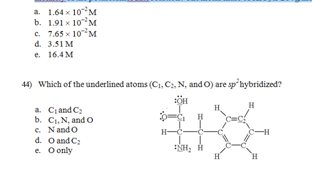
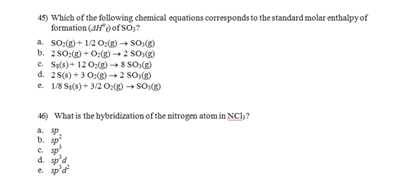
47) All of the following statements concerning molecular orbital (MO) theory are correct EXCEPT:
a. the Pauli exclusion principle is obeyed.
b. Hund's rule is obeyed.
c. electrons are assigned to orbitals of successively higher energy.
d. a bonding molecular orbital is lower in energy than its parent atomic orbitals.
e. the combination of two atomic orbitals creates only one molecular orbital.
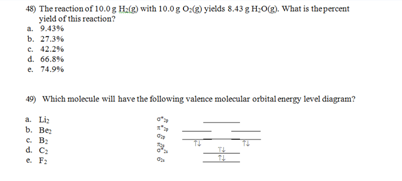
50) Structural isomers are compounds that have
a. the same molar masses, but different elemental composition.
b. have identical structures, but contain different isotopes of the same elements.
c. two or more resonance structures.
d. the same elemental composition, but the atoms are linked in different ways.
e. the same physical properties, but different chemical properties.
51) Which functional group does not contain an oxygen atom?
a. ester
b. ether
c. alcohol
d. carboxylic
e. amine
52) I have enjoyed this class:
a. Yes.
Part 2: Traditional questions. Show your reasoning and use the appropriate number of significant figures
1. Potassium thiocyanate is used in chemical analysis. Its formula is [CNS]- (in no specific order). Based on what you know of the Lewis formulae and the octet rule:
a) draw the three possible arrangements of atoms for this anion, complete with electron dots and in compliance with the octet rule (note: none of the arrangements is triangular);
b) propose the most likely structure of the anion, and state why;
c) indicate specifically which atom (C, N or S) should bear the negative charge on the structure that you proposed in (b.) above.
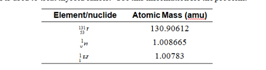
2. Iodine-131 is used to treat thyroid cancer. Use this information for the problem:

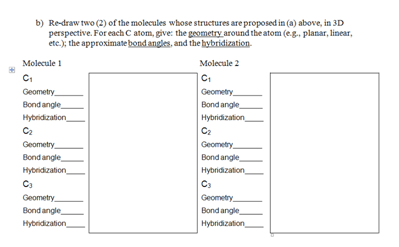
3. Several isomers of organic molecules having the empirical formula C3H6O can be made.
a) Using Lewis structures, draw all the potential forms of organic molecules corresponding to this empirical formula
b) Re-draw two (2) of the molecules whose structures are proposed in (a) above, in 3D perspective. For each C atom, give: the geometry around the atom (e.g., planar, linear, etc.); the approximate bond angles, and the hybridization.
4. Fifty millilitres (50.0 mL) of a 0.133 M sodium iodide solution is mixed with 42.6 mL of a M solution of lead nitrate. A yellow precipitate, lead iodide (PbI2), is obtained.
a) Write the full reaction.
b) Write the net ionic reaction.
c) Calculate the mass (in grams) of the PbI2 obtained from this mixing (the molar mass of PbI2 is 461.0 g/mol). Show your calculations.
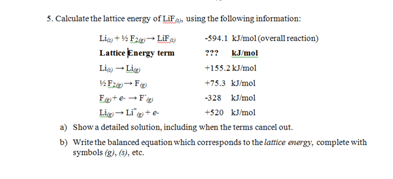
a) Show a detailed solution, including when the terms cancel out.
b) Write the balanced equation which corresponds to the lattice energy, complete with symbols (g), (s), etc.
6. The bond energy (D or ΔHdissociation) is 190 kJ for 1 mol of Br-Br bonds, and 155 kJ for
1 mol of F-F bonds. The heat of vaporization of bromine is + 30.0 kJ/mol, and the standard heat of formation (ΔH°f ) of BrF3(g) is -256 kJ/mol.
a) Write the chemical equation which corresponds to the standard heat of formation of BrF3(g).
b) Write and balance the chemical reactions involved in the processes, and when the terms cancel out.
c) Determine the average energy for a Br - F bond, in kJ/mol. Show your calculations.
