Reference no: EM131128588
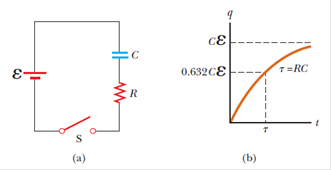
An uncharged capacitor and a resistor are connected in series to a battery, as in Active Figure 18.16a. If e = 12.0 V, C = 5.00 µF, and R = 8.00 105 O, find
(a) the time constant of the circuit,
(b) the maximum charge on the capacitor,
(c) the charge on the capacitor after 6.00 s,
(d) the potential difference across the resistor after 6.00 s, and
(e) the current in the resistor at that time.
Active Figure 18.16 (a) A capacitor in series with a resistor, a battery, and a switch.
|
Find the maximum height through which he can lift the water
: Still thirsty, the Man of Steel repeats his attempt on the Moon, which has no atmosphere. Find the difference between the water levels inside and outside the straw.
|
|
What can be done to address the hazard or exposure
: Identify the Loss Problem, Scope and Magnitude, Hazard and Exposure, Status of Control, Prevention, or Interventions and What can be done to address the hazard or exposure?
|
|
Equal shares of the market for farm-raised catfish
: Four firms have roughly equal shares of the market for farm-raised catfish. The price elasticity of demand for the market as a whole is estimated at -1.5.
|
|
Advantages in relation to situation and culture at pegasus
: Describe 3 models of change. Discuss their advantages and disadvantages in relation to the situation and culture at Pegasus.
|
|
Find the maximum charge on the capacitor
: An uncharged capacitor and a resistor are connected in series to a battery, as in Active Figure 18.16a. If e = 12.0 V, C = 5.00 µF, and R = 8.00 105 O
|
|
Hardware component of a computer
: Which of the following hardware component of a computer can also be called as engine?The sequence of phases, a software goes through from the concept to decommissioning, is called as.
|
|
Depreciation methods-solving for unknowns
: For each of the following depreciable assets, determine the missing amount (?). Abbreviations for depreciation methods are SL for straight line, SYD for sum-of-the-years digits, and DDB for double-declining balance. Please show necessary steps to ..
|
|
What was the new world like after colonization
: what was the new world like after colonization?
|
|
Define uhmwpe and give one potential advantage
: Define UHMWPE and give one potential advantage and one potential disadvantage for using the material in an aerospace application
|