Reference no: EM131129181
1) Does a positive change or a negative change in free energy indicate a favorable (spontaneous chemical event)?
2) If some spontaneous event is endothermic, what must have been the "driving force" behind that event?
3) Given the free energy changes given for coupled reactions that result in the conversion of glucose to glucose 6-phosphate (slide 19). What are the K'eq values for each separate reaction and the overall reaction? How does the mathematical relationship between ?G'° values compare to that between K'eq values?
4) Explain why energy is released when ATP is hydrolyzed and why energy is absorbed when ATP is formed from ADP and Pi.
5) Which residues would you expect to find in a calcium binding site?
6) Would you expect to find the Gln side chain acting as a hydrogen bond donor or acceptor?
7) Why is His often found in the active site of enzyme active sites?
8) Why is protein concentration often measured using A280?
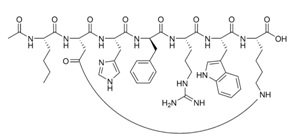
9) Medical Rejuvenation Clinic Australia Pty Ltd (co-owner/director Stephen Dank) sells ?Bremelanotide, which was developed from the peptide hormone Melanotan II, which underwent testing as a sunless tanning agent. In initial testing, Melanotan II did induce tanning, but caused sexual arousal and spontaneous erections in male volunteer test subjects. Based on the figure below:
(a) What amino acids side chains do you recognise, give names, single letter and three letter codes?
(b) does it contain any unnatural amino acids?
(c) Based on the ionisation status of the functional groups in this figure, what can you say about pH?
10) Draw a tripeptide with one peptide bond trans and one cis. Name the amino acids and give their three- and single-letter codes.
11) Draw Ramachandran plots to indicate which region you would find plotted most of the residues in
(a) human growth hormone (hGH) and
(b) the its receptor (hGHR)
12) Medical Rejuvenation Clinic Australia Pty Ltd (co-owner/director Stephen Dank) sells the peptide AOD 9604, sequence YLRIVQCRSVEGSCGF, a peptide from the C-terminus of Human Growth Hormone (HGH), with an added N-terminal tyrosine. When administered by subcutaneous injection would you expect it to maintain a disulfide bond (why/not)? Which residues have the potential to form salt bridges? What overall charge would it carry (show reasoning)?
13) Does disulfide bonding direct folding or does folding direct disulfide bond formation? Discuss the evidence.
14) Ideally, protein folding is encoded in the amino acid sequence alone. Why then do cells have, and need chaperones?
15) Based on what you know about amino acid side chains, devise an experiment to measure the thermal stability of a protein ~150 amino acids long ?.
16) Discuss the role of backbone to backbone hydrogen bonding of the polypeptide in terms of the energetics of protein folding.
17) How do the linear patterns of polar and nonpolar residues in an amino acid sequence relate to how they project from
(i) beta-strands
(ii) alpha- helices?
18) The natural "handedness" of protein secondary structure we observe on Earth (displayed in a Ramachandran plot) is because the naturally occurring amino acids are L-amino acids. What would the Ramachandran plot look like for a planet where the naturally occurring amino acids are D-amino acids?
19) Antibodies are Y-shapes heterotetramers composed of two light chains and two heavy chains . Discuss the secondary, tertiary and quaternary structure of antibodies.
20) Define the term "protein domain" in terms of folding and structure.
21) What are four genetic mechanisms that can give rise to new proteins with new functions?
22) Describe one or more experiments that could test whether a protein contains one or more domain.
23) What is the difference between sequence identity and sequence similarity? Give an example of similarity.
24) What is the general threshold of sequence identity that allows us to make firm predictions about the fold of a protein?
25) If two proteins share the same overall fold, what can you say about their ancestry?
26) Define the term "protein domain" in terms of folding and structure.
27) What are four genetic mechanisms that can give rise to new proteins with new functions?
28) Describe one or more experiments that could test whether a protein contains one or more domain.
29) What is the difference between sequence identity and sequence similarity? Give an example of similarity.
30) What is the general threshold of sequence identity that allows us to make firm predictions about the fold of a protein?
31) If two proteins share the same overall fold, what can you say about their ancestry?
32) Explain the structural features that would account for the different melting points for this 18C fatty acid series: 'Stearic acid 69.6oC'; 'Oleic acid 13.4oC; 'Linoleic acid -5oC; 'Linolenic acid -11oC.
33) How many triaglycerol species are theoretically possible using palmitic acid (16:0) and oleic acid (18:1' 9)? Rank these triaglycerols by their expected melting point.
34) The box cartoon representation depicts the overall organization of a triacylglycerol. The lines between the boxes denote chemical bonds. What are the chemical names for these bonds?
35) Draw equivalent representations of 2 other classes of lipids that also contain glycerol. Annotate the diagram with the chemical names for the different chemical component of these lipids.
36) Draw equivalent representations for 2 main ?groupings of sphingolipids, and again indicate the ?different chemical groups within the sphingolipid.
37) Sodium dodecyl sulfate (SDS) is an anionic detergent open used in biochemistry. Its structure is a single 12C chain with a negatively charged sulfate "head group". What packing arrangement wills 'SDS' adopt in aqueous solution?'
38) Predict the outcomes of mixing solutions of - SDS with free fatty acids and SDS with phospholipids .
39) Integral membrane proteins have a transmembrane domain/sequence that in many (but not all) cases is alpha helical in structure. Knowing that a membrane's phospholipid bilayer is approximately 3nm thick, and that each turn of an alpha helix contains 3.6 amino acids and spans 0.54nm, how many residues are needed in an alpha helix to cross a membrane?
40) Is the following statement true or false? Explain your answer.
"O2 is bigger than Mg2+ and thus will diffuse more slowly across a lipid bilayer."