Reference no: EM13988488
1. Rf Values for TLC Plate #1
2. Complete the table below. If more than one spot shows up for a sample, include both Rf values.
| Sample |
Rf Values |
| Tylenol |
|
| Motrin |
|
| acetaminophen |
| ibuprofen |
Understanding and Conclusions
3. Draw the structures of the organic active ingredients in Tylenol and Motrin, be sure to clearly indicate which is which. Explain your choice in ONE or TWO SENTANCES.
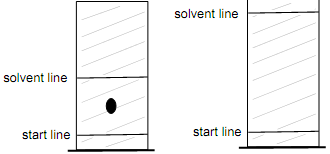
4. A sample is run on a TLC plate and gives a single spot shown below, left. However, the solvent was not allowed to run up the plate very far, and so the experiment was repeated with a much larger solvent distance, as shown on the right below. Draw the spot be on the plate on the right using the plate on the left as a guide.
5. A TLC experiment is performed on phenol and aniline, structures given below. Two spots are observed. X and Y. Decide which spot, X or Y is phenol and which is aniline. Give a BRIEF explanation in terms of intermolecular forces (you are not allowed to simply use the term "polarity").
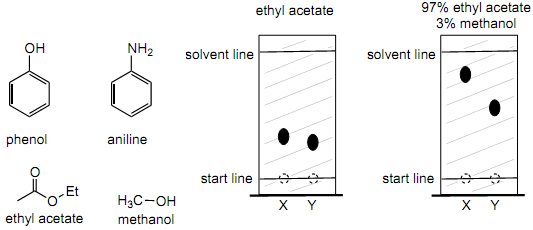
6. Referring back to question 8 above, in the TLC plate on the left the eluting mobile phase solvent was ethyl acetate, in the plate on the right it was 97% ethyl acetate and 3% methanol. Explain why the Rf values in the plate on the right are larger than those in the plate on the left. Your explanation must be in the terms of intermolecular forces, you are not allowed to only use the word "polarity".
7. You mark the TLC plate with a pencil and not a pen. Why is this?