Reference no: EM131307550
1) What is the difference between closed and isolated systems?
2) Is it true that thermodynamic equilibrium is reached very rapidly (a few seconds or less) in any system? Justify your answer or provide examples that support it.
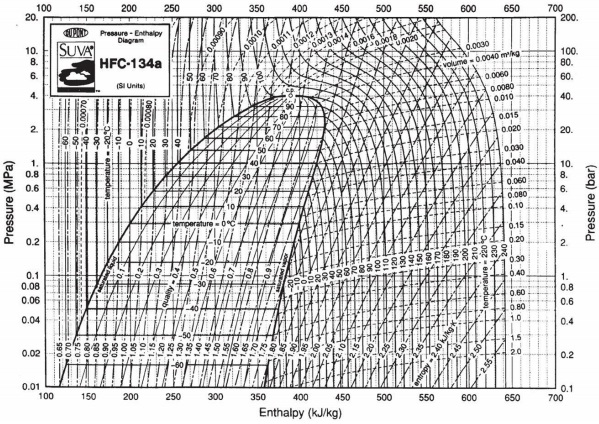
3) A continuous flow of 1 kg/s of refrigerant HFC-134a at a pressure of 4 MPa and temperature of 120oC enters a valve, operating at steady-state, from which it leaves at 0.1 MPa and 40oC. What is the heat transfer rate in the valve?
4) 22.8 kg of n-octane (C8H18, molar mass=114 g/mol) and the stoichiometric amount of oxygen are fed to a closed, isothermal reactor (700 Kelvin) of constant pressure (10 bar) and variable volume that initially contains no other chemicals. The reaction stops when all n-octane is consumed (final state).
a) Find the number of moles of oxygen, carbon dioxide, and water in the final state.
b) Assume the mixture in the final state is an ideal gas. Find its volume.
5) A continuous ideal gas stream with flow rate equal to 20 moles/s at 900 K and 10 bar enters an adiabatic reversible turbine operating at steady-state, from which it exits at 2.5 bar. The molar heat capacity at constant pressure of this gas is equal to 30 J/(mol.K).
a) Starting from the general form of the mass, energy, and entropy balances, cancel out what is applicable to obtain working expressions for the situation described. Neglect changes in kinetic and potential energies.
b) Find the rate of entropy generation in the turbine.
c) Find the gas temperature after the turbine.
d) Find the power obtained in the turbine.
6) An external wall that is adiabatic, rigid, and impermeable isolates a composite system from the surroundings. An internal wall separates the two subsystems A and B that form the composite system. Subsystem A contains ideal gas "a" whose molar heat capacity at constant pressure is equal to 21 J/(mol.K). Subsystem B contains ideal gas "b" whose molar heat capacity at constant pressure is equal to 29 J/(mol.K). In the initial state, the internal wall separating two subsystems A and B is adiabatic, rigid, and impermeable, and each subsystem has volume equal to 1 m3 and pressure equal to 5 bar. The initial temperatures in subsystems A and B are equal to 300 K and 600 K, respectively.
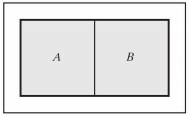
a) Find the number of moles present in each subsystem.
b) Assuming the internal wall becomes diathermal, rigid, and impermeable, find the temperature and pressure in each subsystem at equilibrium.
c) Assuming the internal wall becomes diathermal, moveable, and impermeable, find the temperature and pressure in each subsystem at equilibrium.