Reference no: EM131260283
Problem 1 - During a soft drink bottling process, the soft drink is carbonated using CO2. Each 1-liter bottle of soft drink requires 0.62 g of CO2 for the desired level of carbonation. The CO2 is supplied from very high pressure tanks held in a "hot box" at 61°C. The fully charged tanks come from the vendor at 3735 psig, and are considers "empty" and returned to the vendor for refilling when the pressure gauge reads 60 psig.
Critical properties of CO2: Tc = 304.2 K Pc = 72.9 atm
a. What mass fraction of the CO2 originally supplied in each tank is unused when the tank is returned?
b. If the tank volume is 2 ft3, how many 1-liter soft drink bottles can be carbonated by each tank?
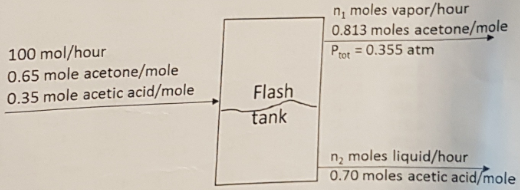
Problem 2 - In a single stage flash operation, the liquid feed mixture of acetone and acetic acid at high pressure and temperature flows to a flash tank where the pressure is rapidly dropped, as shown in the figure below. The liquid and gas phase exit the tank in equilibrium with each other at total pressure of 0.355 atm absolute. A sample of the gas stream is analyzed and determined to contain 0.813 mol fraction acetone.
a. Determine the temperature of the streams exiting the flash, and the partial pressures of both components in the gas phase.
b. Determine the total molar flow rates n1 and n2 of the streams exiting the flash tank.
Antoine Equation constants: log10p* = A - (B/T+C), p* in mm Hg, T in oC
|
Compound
|
Formula
|
A
|
B
|
C
|
|
Acetic acid
|
C2H4O2
|
7.38782
|
1533.313
|
222.309
|
|
Acetone
|
C3H6O
|
7.11714
|
1210.595
|
229.664
|
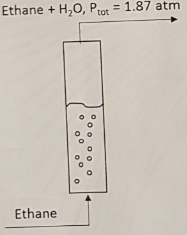
Problem 3 - To study hydrocarbon solubilities in water related to possible groundwater contamination, the experimental apparatus shown below is used. In this experiment, ethane (C2H6) is sparged, or bubbled, through a column of water such that the exiting gas stream is in equilibrium with the liquid. The absolute pressure of the exiting stream is measured to be 1.87 atm, and the temperature of the liquid is measured to be 18.5°C. A sample of the liquid is analyzed and found to contain 0.124 g ethane/liter.
Determine the Henry's law constant He (atm/mol fraction) for ethane in water at 18.5°C.
|
Develop an expectation for ticket revenue
: The auditors for Weston University are conducting their audit for the fiscal year ended December 31, 2015. Specifically, the audit firm is now focusing on the audit of revenue from this season’s home football games. Based on the information above, de..
|
|
Determine the smallest permissible dimension
: A torque of 400 lb ft is applied to the square tube with constant 0.10-in. wall thickness. Determine the smallest permissible dimension a if the shear stress is limited to 6500 psi.
|
|
Which dispatching rule has the best score for flow time
: Which dispatching rule has the best score for flow time? - Which dispatching rule has the best score for utilization metric?
|
|
Create a java program
: Using arrays create a java program were a company that pays its sales staff on a commission basis. Each employee receives $500 per week plus 6.5% of their sales. For example, a salesperson who sells $10,000 worth of products will get a salary of 5..
|
|
Determine the temperature of the streams exiting the flash
: In a single stage flash operation, the liquid feed mixture of acetone and acetic acid at high pressure and temperature flows to a flash tank where the pressure is rapidly dropped, Determine the temperature of the streams exiting the flash, and the ..
|
|
How the job skills can lead to improved job performance
: Discuss how these job skills can lead to improved job performance. Explain how Organizational Behavior can aid you in decision-making and problem-solving. Predict the consequences of unethical behavior in the workplace.
|
|
Define the price elasticity of demand
: Define the law of diminishing returns. Why is this law considered a short-run phenomenon and State the general meaning of elasticity as it applies to economics. Define the price elasticity of demand.
|
|
Find the largest allowable torque that can be applied
: The cross section of a brass tube is an equilateral triangle with a constant wall thickness, as shown in the figure. If the shear stress is limited to 8 ksi and the angle of twist is not to exceed 2° per foot length, determine the largest allowabl..
|
|
Analyze the consumer decision making process for automobiles
: Identify the best target market segments for the Ecostar. Analyze the consumer decision making process for automobiles in the segment you have identified for the Ecostar.
|