Reference no: EM13924259
Fundamental of Nanotechnology-Self Assembly Phenomena
Problem 1
The micellization of surfactant can be represented by a stepwise association process given by the equilibrium reaction below:
S1 + Sn-1 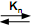 sn
sn
For a micellization process with identical equilibrium constant for each step, show that the C, M, W and the polydispersity index are given by the expression below:
(Note: please refer to your notes for more information)
(a) C = |S1|/(1 - X) (c) W = [S1](1 + X)/(1-x3)
(b) M = [S1]/(1-x2) (d) N-w / N-n = 1 + X/(2 - X)2
Where C, M, W, and X are given by:
C = 1Σn[Sn] M = 1Σnn[Sn] w= 1Σnn2[Sn] X = K[S1]
Problem 2
(a) The following surface tension were measured for aqueous solutions of the nonionic surfactant CH3(CH2)9(OCH2CH2)50H at 25 °C
|
C(10-4mo1/dm-3)
|
0.1
|
0.3
|
1.0
|
2.0
|
5.0
|
8.0
|
10.0
|
20.0
|
30.0
|
|
y (mN/m)
|
63.9
|
56.2
|
47.2
|
41.6
|
34.0
|
30.3
|
29.8
|
29.6
|
29.5
|
Determine the critical micelle concentration and calculate the area occupied by each adsorbed surfactant molecule at the critical micelle concentration.
(b) The critical micelle concentration of nonionic surfactant in water varies with temperature as follows:
|
Temperature (°C)
|
15
|
20
|
25
|
30
|
40
|
|
CMC (mmol/dm3)
|
21.9
|
20.8
|
19.9
|
19.1
|
17.8
|
Determine an enthalpy of micellization according to the phase separation model.
|
What is involved in a macroanalysis of the industry earning
: What is involved in a macroanalysis of the industry earnings multiplier? What are the steps in the microanalysis of an industry earnings multiplier?
|
|
Two other learners, company evaluation
: Surf Shop Comparison. Access the web sites of Ron Jon Surf Shop (http://www.ronjonsurfshop.com/) and Hilo Hattie a similar retailer in Hawaii. Explain how the two are similar and how they are different. Can you find elements of planning, organizin..
|
|
How many units should the firm produce to maximize profit
: A monopoly firm estimates the demand function (curve) for its output to be: Q = 1624 - 0.25P, and its total variable cost function as TVC = 24Q - 4Q2 + 1/3Q3, Where Q is output How many units should the firm produce to maximize profit
|
|
Scores for any particular continuous dependent variable
: ANOVA examines statistically significant group differences, particularly when we have more than two groups to contrast with each other, on the scores for any particular continuous dependent variable. Is this statement?
|
|
Determine an enthalpy of micellization
: Determine an enthalpy of micellization according to the phase separation model - Determine the critical micelle concentration and calculate the area occupied by each adsorbed surfactant molecule at the critical micelle concentration.
|
|
Description and job specification for a starbucks employee
: Case Study: Starbucks' Structure. Review the case study found in Chapter 3 of your text titled "Starbucks' Structure" and write a paper that answers the four case study questions listed below in narrative form using APA format. Provide supporting ..
|
|
Types of tonicity gradients
: In this exercise two of the three types of tonicity gradients were observed. Suggest an experiment to provide an example of the third type of tonicity gradient in action.
|
|
How does incorporation by reference affect the enforcement
: How does incorporation by reference affect the enforcement of NFPA guidelines? Do you believe incorporating certain guidelines is a good approach by OSHA
|
|
Describe their significance to society, health
: Discoveries in DNA, cell biology, evolution, biotechnology have been among the major achievements in biology over the past 200 years with accelerated discoveries and insights over the last 50 years.
|