Reference no: EM131128714
Assignment. A terrible plague of H3N2 influenza has begun to decimate the earth's population. You convince your boss at your pharmaceutical company to let you work on developing a new neuraminidase inhibitor to combat this new strain. You make several hundred potential inhibitors and assay them with the neuraminidase isolated from this new strain. Frustratingly, none of the compounds has the required Ki of less than 2 µM to effectively inhibit this enzyme. Finally, in desperation attempt, you decide to make one last compound. You then assay this compound with the viral neuraminidase (which is used in the assay at 1 nM concentration).
After doing a complete study at various substrate and inhibitor concentrations, you arrive at the following data. The numbers inside the box are the reaction rates measured in nM/s. This table is an embedded MS excel file that can easily be copied into Microsoft Excel by cutting and pasting.
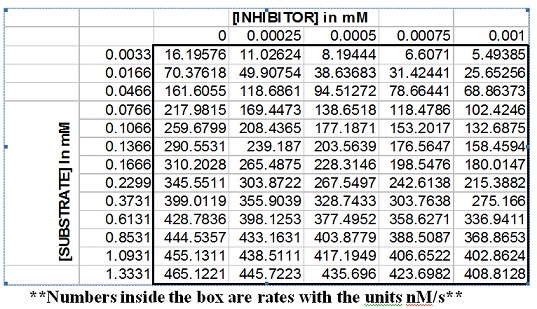
1. Using the data given, determine the Vmax, kcat, and Km for the substrate with no inhibitor present (Be sure to include units!). Attach a rate vs. [S] plot and a Lineweaver-Burk plot below. Describe how you determined these parameters. Show equations.
2. Perform a complete inhibitor study (rate vs. [S] plots at each concentration of inhibitor, your plots should each have 5 lines) using Excel and the data within the table. Attach the rate vs. [S] and Lineweaver-Burk plots below and label each curve with the inhibitor concentration for the data shown by that curve.
3. Answer the following question. What type of inhibitor is this compound.
4. Using a secondary plot like we described in class, determine the Ki for this compound. Attach the secondary plot below and describe how you determined Ki from this plot.
5. Did your compound save the world from this outbreak? true or false.
|
Which of processes does not require energy input
: Transcription, translation, replication, synthesis of phospholipids, or do they all require energy?- Which of these processes does not require energy input?
|
|
Produces three kinds of table wine-a blush
: The Otter Creek Winery produces three kinds of table wine-a blush, a white, and a red. The win- ery has 30,000 pounds of grapes available to produce wine this season. A cask of blush requires 360 pounds of grapes, a cask of white requires 375 poun..
|
|
What hose diameter is needed
: For a flow rate of 1.5 L/s, what hose diameter is needed? Use H = 5m and h = 0.5m. Assume all head loss occurs in the tube. Use the rigorous approach to calculate D.
|
|
Describe how you determined ki
: Using a secondary plot like we described in class, determine the Ki for this compound. Attach the secondary plot below and describe how you determined Ki from this plot.
|
|
An american low-cost airline headquartered
: JetBlue Airways is an American low-cost airline headquartered in New York City. Its main base is John F. Kennedy International Airport. JetBlue's revenue in 2002 was $635.2 mil- lion. By 2009, revenue had increased to $3,290.0 million.
|
|
Provide recommendations for preventing spills or releases
: Discuss how you applied each of the steps in the GEBMO process and what risks you identified. Provide recommendations for preventing spills or releases. Discuss response actions required in the event of a spill or release.
|
|
Model rocket engine a thrust curve as shown
: Challenge Homework 5 Physics 211 Due 8/4/2015 1. A B-4 Estes model rocket engine has a thrust curve as shown. Putting this engine in a 100.0 g rocket (including the weight of the engine and discounting the loss in mass as the fuel burns), and firi..
|
|
Based on everything you have learned about quality
: Based on everything you have learned about quality, write your own definition of what quality is. What do you think are the most important things that your organization should do in order to improve quality? What are the biggest barriers to quality i..
|