Reference no: EM13772250
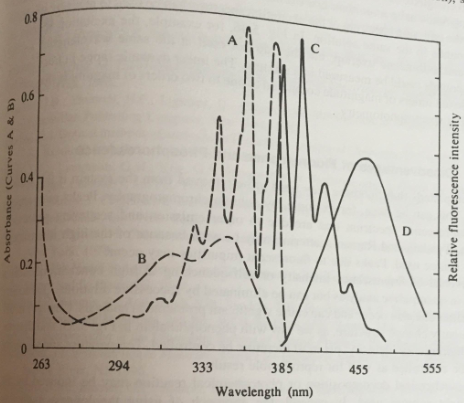
1. From the emission spectra of quinine and anthracene, pick a wavelength that will permit you to determine quinine in a mixture at quinine and anthracene. Do the same for anthracene. Can you use the excitation spectra to distinguish between the two compounds?
Explain.
2. A solution contains 1.0 mg of KMn04/L. When measured in a 1.00 cm cell at 525 nm, the transmittance was 0.300. When measured under similar conditions at 500 nm, the transmittance was 0.350.
(a) Calculate the absorbance A at each wavelength.
(b) Calculate the molar absorptivity at each wavelength.
(c) What would T be if the cell length were in each case 2.00 cm?
(d) Calculate the absorptivity (if concentration is in mg/L) for the solution at each wave length.
3. An amount of 0.200 g of copper is dissolved in nitric acid. Excess ammonia is added to form Cu(NH3)42±, and the solution is made up to 1 L. The following aliquots of the solution are taken and diluted to 10.0 mL: 10.0, 8.0, 5.0, 4.0, 3.0, 2.0, and 1.0 mL. The absorbances of the diluted solution were 0.500, 0.400, 0.250, 0.200, 0.150, 0.100, and 0.050, respectively. A series of samples was analyzed for copper concentration by forming the Cu(NH3),21+ complex and measuring the absorbance. The absorbances were (a) 0.450, (b) 0.300, and (c) 0.200. What were the respective concentrations in the three copper solutions? If these three samples were obtained by weighing out separately (a)1000 g, (b) 2.000 g, and (c) 3.000 g of sample, dissolving
4. The following data were obtained in a external standard calibration for the determination of iron by measuring the transmittance, at 510 nm and 1.00 cm optical path. of solutions of nthrohnet to give a red-colored complex
(a) Calculate A, the absorbance, for each solution and plot A against concen-tration of iron. (You can do this using a spreadsheet program very easily.) Does the system conform to Beer's Law over the entire concentration range? (b) Calculate the average molar absorptivity of iron when it is deter-mined by this method. (c) Plot (100 - %T) against log concentration (Ringbom method). (1) What are the optimum concentration range and the ercent relative error per I % transmittance error) in maximum accuracy will the relative analysis this range? (2) Over what concentration rang error per 1% transmittance error not exceed 5%?
5. In prepanng a calibration curve for the determination of methyl ethyl ketone (MEK) solutions with different concentrations of MEK were prepared in chloroform. The absorbance at the Co. stretching frequency was measured.
The measured absorbance A for each solution is given. A blank (the pure solvent) had an absorbance = 0.00.
|
MEK concentration (%)
|
Absorbance
|
|
2
|
0.18
|
|
4
|
0.36
|
|
6
|
0.52
|
|
8
|
0.64
|
|
10
|
0.74
|
(a) Does the relationship between A and sample concentration deviate from Beer's Law? (b) Several unknown samples containing MEK were measured at the same wavelength as that used for the calibration curve. The results were as follows:
Sample Absorbance
A 0.27
B 0.44
C 0.58
D 0.69
What were the concentrations of MEK in the solutions A-D?
|
Importance of theory application and self-efficacy
: How effective would your program be if it wasn't guided by a theoretical foundation?
|
|
Conventional level of personal moral development
: Which of the following leadership mindset emphasizes tight top-down control, employee standardization and specialization, and management by impersonal measurement and analysis?
|
|
Write a program that inputs an integer in the range
: Write a program that inputs an integer in the range [-32767, 32767] from the Serial Monitor and checks to see if that number is a prime number. If the number is a prime number, have the LED on pin 13 turn on. If not, then turn the LED off.
|
|
Electrons to silver in a redox reaction
: Of the metals Au, Fe, Na, and Cd, which will not give up its electrons to silver in a redox reaction
|
|
Calculate the molar absorptivity at each wavelength
: Calculate the absorbance A at each wavelength, calculate the molar absorptivity at each wavelength and what would T be if the cell length were in each case 2.00 cm - What were the concentrations of MEK in the solutions A-D?
|
|
What was your favorite assigned reading and discussion
: What was your favorite assigned reading and/or discussion, and why? Do you feel that you have become a better reader and writer through this course? If so, in what ways? If not, why?
|
|
The advantages and disadvantages of social networking
: Analyze how the university might integrate at least two social media and networking technologies to accomplish their goals. Your analysis must cover the advantages and disadvantages of social networking. The president of the university also needs ..
|
|
Identification of the dimension of quality
: Identify the article you have selected in the first line of your posting and note the related quality dimension (effectiveness).Briefly summarize the article.
|
|
What were the factors that shaped indra nooyi as a leader
: What were the factors that shaped Indra Nooyi as a leader? What are the factors that could make Nooyi change her decision about corporate sustainability?
|