Magnetic properties : Most of the transition elements and their compounds show paramagnetism. Paramagnetism increases first in any transition element series, and then decreases. Maximum paramagnetism is obsreved around the middle of the series. The paramagnetism is described in the Bohr Magneton (BM) units. The paramagnetic moments of some ordinary ions of the first transition series are given below in the Table
Explanation : A substance which is attracted by magnetic field is called paramagnetic substance. The substances which are being repelled by magnetic field are, termed as diamagnetic substances. Paramagnetism is because of the presence of unpaired electrons in ions, atoms or molecules.
Magnetic moment of any of the transition element or its compound/ion can be given by (assuming no contribution from orbital magnetic moment).
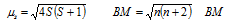
here, S is the total spin (n * s): n is number of the unpaired electrons and s is equal to ½ (representing spin of an unpaired electron).
From equation given above, the magnetic moment 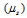 increases with the increase in the number of unpaired electrons.
increases with the increase in the number of unpaired electrons.
Magnetic moments of some ions of the 3d-series elements
|
Ion
|
Outer configuration
|
No. of unpaired electrons
|
Magnetic moment (BM)
|
|
|
|
|
Calculated
|
observed
|
|
Sc3+
|
3d0
|
0
|
0
|
0
|
|
Ti3+
|
3d1
|
1
|
1.73
|
1.75
|
|
Ti2+
|
3d2
|
2
|
2.84
|
2.86
|
|
V2+
|
3d3
|
3
|
3.87
|
3.86
|
|
Cr2+
|
3d4
|
4
|
4.90
|
4.80
|
|
Mn2+
|
3d5
|
5
|
5.92
|
5.95
|
|
Fe2+
|
3d6
|
4
|
4.90
|
5.0-5.5
|
|
Co2+
|
3d7
|
3
|
3.87
|
4.4-5.2
|
|
Ni2+
|
3d8
|
2
|
2.84
|
2.9-3.4
|
|
Cu2+
|
3d9
|
1
|
1.73
|
1.4-2.2
|
|
Zn2+
|
3d10
|
0
|
0
|
|
In d-obitals belonging to a specific energy level, there can be at maximum five unpaired electrons in d5 cases. Therefore, paramagnetism in any transition series first increases, reaches a maximum value for d5 cases and then decreases thereafter.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Magnetic Properties questions? Magnetic Properties topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Magnetic Properties related problems. We provide step by step Magnetic Properties question's answers with 100% plagiarism free content. We prepare quality content and notes for Magnetic Properties topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours