Benzene (C6H6)
Benzene is the first member of arenes. It was first discovered by Faraday (1825) from whale oil. Mitscherllich (1833) obtained it by distillating benzoic acid with lime. Hofmann (1845) got it from coal tar, which is also a commercial source of benzene.
Structure of benzene : Benzene has a special structure, which is although unsaturated even then it generally behave as a saturated compound.
(i) Kekule's structure : Kekule states that in benzene 6-carbon atoms placed at corner of hexagon and bonded with hydrogen and double bond present at alternate position.
(a) Evidence in favor of Kekule's structure
· Benzene combines with 3 molecules of hydrogen or three molecules of chlorine. It also collides with 3 atoms of ozone to form triozonide. These reactions show the existence of three double bonds.
· Studies on molecular refraction and magnetic rotation show the presence of three double bonds and a conjugated system.
· The synthesis of benzene from three atoms of acetylene also favour's Kekule's structure
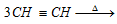
· Benzene provides cyclohexane by reduction with hydrogen.
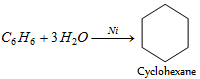
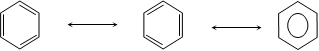
(b) Objections against Kekule's relation
· Unusual stability of benzene.
· According to Kekule, two ortho disubstituted products are possible. But in examples only one ortho disubstituted product is known.
· Heat of hydrogenation of benzene is 49.8 kcal/mole, whereas practical value of heat of hydrogen ation of benzene is 85.8 kcal/mole. It defines resonance energy is 36 kcal/mole.
· C-C bond length in benzene are same, (although it has 3 double bonds and 3 single bonds) and has 1.39 Å.
Kekule explained this objection by proposing that double bonds in benzene ring were continuously oscillating between two adjacent positions.
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Benzene questions? Benzene topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Benzene related problems. We provide step by step Benzene question's answers with 100% plagiarism free content. We prepare quality content and notes for Benzene topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours