Alkanes [Paraffines]
"Alkanes are saturated hydrocarbon containing only carbon-carbon single bond in their molecules."
Alkanes are less reactive so known as paraffins; because under normal conditions alkanes do not react with acids, bases, oxidising agents and reducing agent.
General formula : CnH2n+2
Examples are CH4, C2H6, C3H8,
General Methods of preparation
(i) By catalytic hydrogenation of alkynes and alkenes (Sabatie and sanderen's reaction)
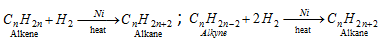
- Methane is not created by this process
(ii) Birch reduction :
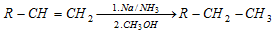
(iii) From alkyl halide
(a) By reduction : 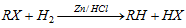
(b) With hydrogen in existence of pt/pd : 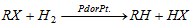
(c) With HI in existence of Red phosphorus : 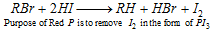
(iv) By Zn-Cu couple :
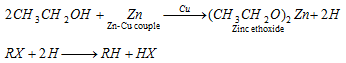
(v) Wurtz reaction :
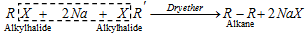
- R-Br or RI preferred in this reaction. The total result in that reaction is the formation of even no. of carbon atoms in molecules.
(vi) Frankland's reaction :
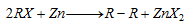
(vii) Corey-house synthesis
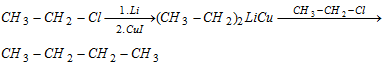
- Reaction is suitable for odd number of Alkanes.
(viii) From Grignard reagent
(a) By action of acidic 'H' :

(b) By reaction with alkyl halide :
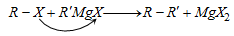
(ix) From carboxylic acids
(a) Laboratory method [Decarboxylation reaction or Duma reaction]
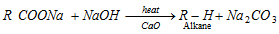
- NaOH and CaO is in the ratio of 3 : 1. (Sodalime)
(b) Kolbe's synthesis :
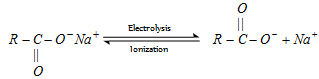
At anode [Oxidation] :
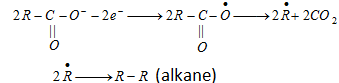
At cathode [Reduction] :
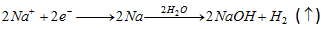
- Both free radical and ionic process are required in this reaction.
(c) Reduction of carboxylic acid :
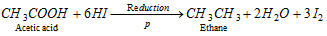
(x) By reduction of alcohols, ketones, acid derivatives or aldehyde
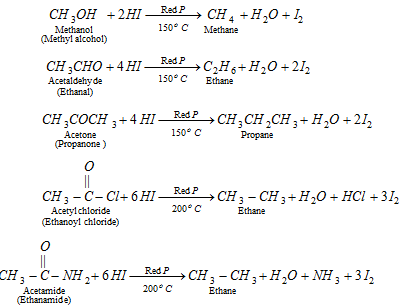
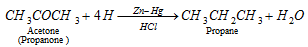
q Aldehyde and ketones when reduced with conc. HCl and amalgamated zinc also yield alkanes.
Clemmenson reduction :
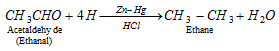
- Ketones and aldehydes (>C = O) may be converted to hydrocarbon in presence of excess of hydrazine and sodium alkoxide on heating.
Wolff-kishner reduction :
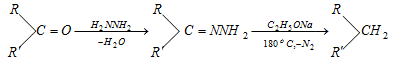
(xi) Hydroboration of alkenes
(a) On treatment with acetic acid
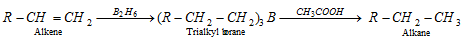
(b) Coupling of alkyl boranes by incomes of silver nitrate
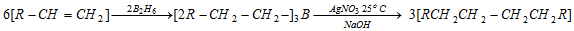
Email based Chemistry assignment help - homework help at Expertsmind
Are you searching chemistry expert for help with Alkanes questions? Alkanes topic is not easier to learn without external help? We at www.expertsmind.com offer finest service of Chemistry assignment help and chemistry homework help. Live tutors are available for 24x7 hours helping students in their Alkanes related problems. We provide step by step Alkanes question's answers with 100% plagiarism free content. We prepare quality content and notes for Alkanes topic under chemistry theory and study material. These are avail for subscribed users and they can get advantages anytime.
Why Expertsmind for assignment help
- Higher degree holder and experienced experts network
- Punctuality and responsibility of work
- Quality solution with 100% plagiarism free answers
- Time on Delivery
- Privacy of information and details
- Excellence in solving chemistry queries in excels and word format.
- Best tutoring assistance 24x7 hours